Health-Care Workers & Nursing Home Residents First In Line For Vaccine, CDC Panel Votes
Tyler Durden
Tue, 12/01/2020 – 17:36
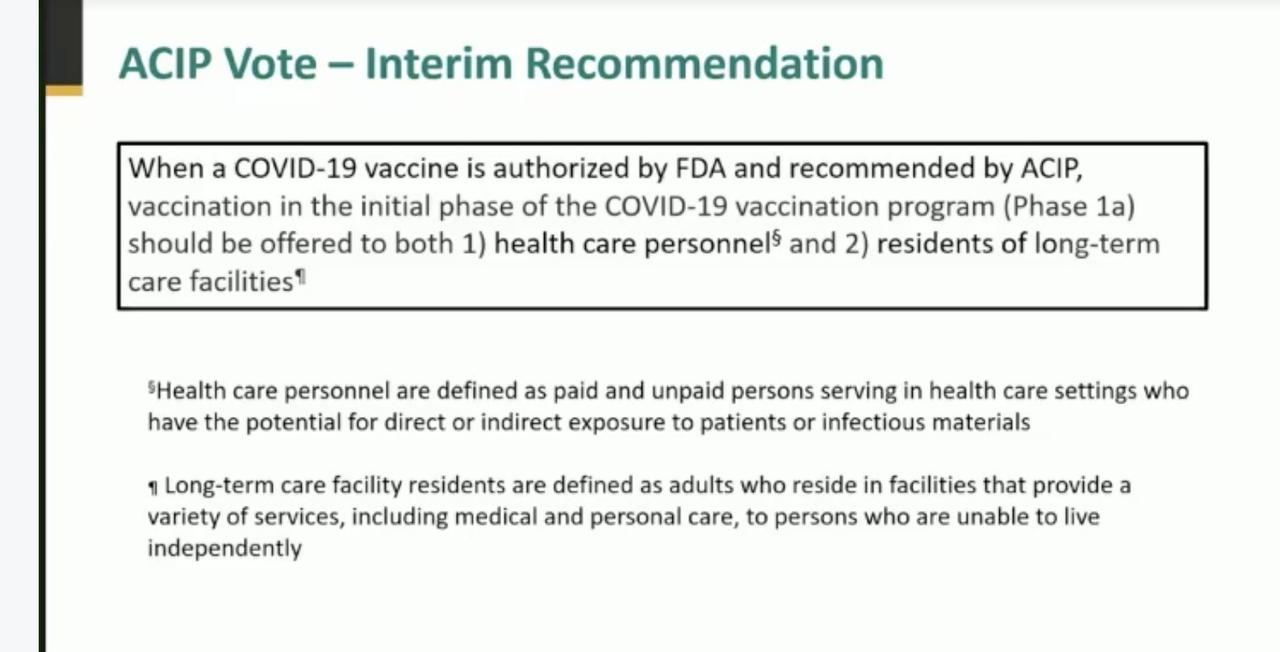
By a vote of 13-1, a Centers for Disease Control and Prevention (CDC) panel (The Advisory Committee on Immunization Practices or the ACIP). has decided that health-care workers and long-term care facility residents will receive the first COVID-19 vaccine doses once it’s cleared for public use.
“Long term care facility residents are defined as adults who reside in facilities that provide a variety of services, including medical and personal care, to persons who are unable to live independently,” the CDC said.
“Health care personnel are defined as paid and unpaid persons serving in health care settings who have the potential for direct or indirect exposure or infectious materials.”
They also discussed “sub-prioritization” within each population. In other words, it’s not simply “all healthcare workers come first.” The healthcare workers who come into contact with infectious diseases (or suspected COVID) would come first.
The CDC will take the recommendation into account and provide guidance to the states to assist governors (who will make the ultimate choice) in their decisions about vaccine distribution priorities.
This decision, if followed by CDC means that other high priority groups will have to wait, including people with underlying medical conditions, essential workers such as cops/firefighters, and elderly people not in nursing homes.
As CNBC reports, there are roughly 21 million health-care workers and 3 million long-term care facility residents in the United States, according to a presentation during the CDC’s Advisory Committee on Immunization Practices, an outside group of medical experts that advises the agency.
Dr. Nancy Messonnier, director of the CDC’s National Center for Immunization and Respiratory Diseases, said most states and local jurisdictions expect it to take three weeks to vaccinate all of their health-care workers.
“Within 24 hours, maybe at most 36 to 48 hours, from the approval, the vaccine can be in people’s arms,” Moncef Slaoui, a former GlaxoSmithKline executive, said at an event conducted by The Washington Post newspaper.
Pfizer and Moderna’s vaccines require two doses about a month apart.
“We really need to make patients aware that this is not going to be a walk in the park,” Fryhofer said during a virtual meeting with the Advisory Committee on Immunization Practices, or ACIP, an outside group of medical experts that advise the CDC. She is also a liaison to the committee.
“They are going to know they had a vaccine. They are probably not going to feel wonderful. But they’ve got to come back for that second dose.”
Critically, the single vote against the recommendation came from Dr. Helen Talbot of Vanderbilt University, who said she was worried that the vaccine had not been studied in residents of long-term care facilities.